Purpose of the flight and payload description
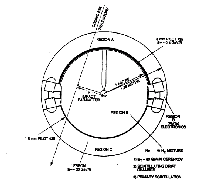
Is a complex detector of cosmic rays. The detector is shown schematically at left. A high energy cosmic ray transversing regions A, B, and C generates several signals: A Pilot Cerenkov signal from regions A, and C; a scintillation signal in region B, and an amplified scintillation when the liberated charge reaches the high electric field region close to the central electrode. Cosmic rays exceeding 20 Gev/a also liberate a freon Cerenkov signal from region C, and those exceeding 70 GeV/a a gas Cerenkov signal from region B. All these signals are optically mediated and obtained from the 40 photomultiplier tubes in the instrument.
Although the instrument was destroyed on landing, data were telemetered for about 24 hours whilst at float altitude.
Details of the balloon flight
Balloon launched on: 9/29/1993 at 17:37 utc
Launch site: Scientific Flight Balloon Facility, Fort Sumner, (NM), US
Balloon launched by: National Scientific Balloon Facility (NSBF)
Balloon manufacturer/size/composition: Zero Pressure Balloon SF3-424.37-080-NSCHR-X-ST
Balloon serial number: W29.47-2X-05
Flight identification number: 372N
End of flight (L for landing time, W for last contact, otherwise termination time): 9/30/1993 at 18:27 utc
Balloon flight duration (F: time at float only, otherwise total flight time in d:days / h:hours or m:minutes - ): ~ 24 h
Landing site: 10 miles SE of Woodward, Oklahoma, US
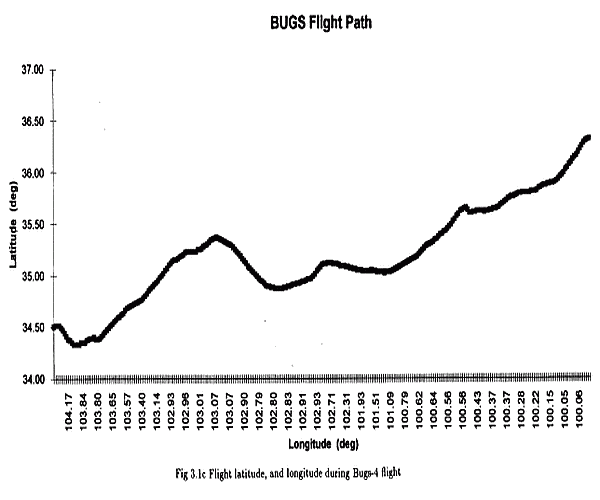
Bugs-4 was launched by dynamic method at 17:57 (GMT) its trajectory was complex, but generally due east and after 22,8 hours at float descent operations started: the balloon was severed from the instrument then the parachute opened at the appropriate altitude. The entire descent phase lasted 52 minutes.
Once on ground the parachute was cut away from the instrument.
The crew in the chase plane observed that the instrument struck a power line, and it is believed that sparks from the severed line ignited the scrub. The fire was spread by strong ground winds. A chemist with the recovery crew indicated there was little natural fuel for the fire, but the ethafoam 220 insulation caught fire, and the instrument was consumed. Tests with small samples of ethafoam 220 indicate it ignites easily, and evolves many joules of heat as it burns.
(Extracted from a scientific paper and completed with the kindly help of NSBF's Bob Redinger)
External references
- A measurement of the energy spectra of cosmic rays from 20 to 1000 GeV per AMU Topical Semiannual Report; 1 Apr. 1993 - 31 Mar. 1994
- An instrument to measure the charge, and energy spectrum (20-1000 GeV/a) of the cosmic ray species oxygen to iron Topical Semiannual Report No. 2; 1 Mar. - Sep. 1994
- An Instrument to Measure the Energy Spectra of Cosmic Rays from 20 to 100 GeV per Nucleon 22nd International Cosmic Ray Conference. 1991. Volume 2, p.587
- Measurement of the Energy Spectra of Cosmic Rays from 20 to 1000 GeV Per Amu Final Report, NASA Marshall Space Flight Center; 1997
- NASA Balloon Flights (19891998) in NASA Historical Data Book, Vol. VII: NASA Launch Systems, Space Transportation, Human Spaceflight, and Space Science, 1989-1998
- Performance of the BUGS IV Spherical Drift Chamber 24th International Cosmic Ray Conference, 1995, Vol. 3, p.637
- Results from the Flight of BUGS-4 24th International Cosmic Ray Conference, Vol. 3, p.637
If you consider this website interesting or useful, you can help me to keep it up and running with a small donation to cover the operational costs. Just the equivalent of the price of a cup of coffee helps a lot.